Mechanism of interaction of Arginase-1 inhibitors
Understanding the impact of different assay conditions
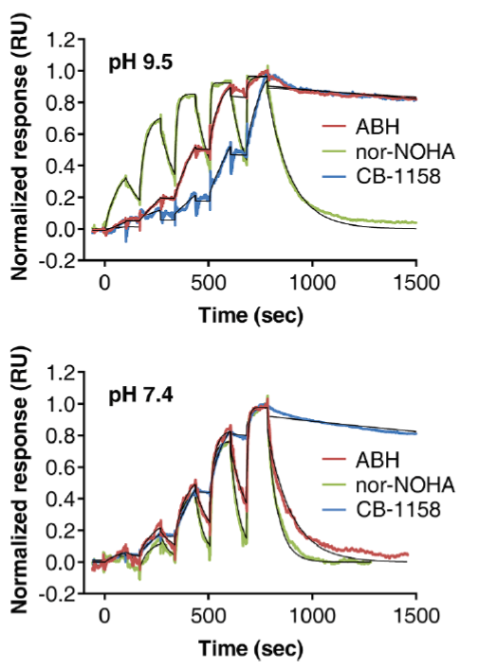
| Inhibition assay | Thermal shift assay | SPR assay | |||||
| Inhibitor | IC50 (nM) | ΔTm (°C) | ka (M-1 s-1) | kd (s-1) | KD (nM) | τ (s) | |
| pH 9.5 | ABH | 22 | 3.5 | 5.1 × 103 | 1.4 × 10-4 | 27 | 7200 |
| nor-NOHA | 109 | 4.7 | 3.6 × 104 | 6.2 × 10-3 | 173 | 160 | |
| CB-1158 | 132 | 5.6 | 1.3 × 103 | 9.2 × 10-5 | 72 | 11000 | |
| pH 7.4 | ABH | 184 | 4.5 | 3.6 × 104 | 9.9 × 10-3 | 797 | 100 |
| nor-NOHA | 59 | 4.8 | 3.6 × 104 | 1.9 × 10-2 | 1497 | 54 | |
| CB-1158 | 8.6 | 8.1 | 4.8 × 103 | 1.8 × 10-4 | 38 | 5500 | |