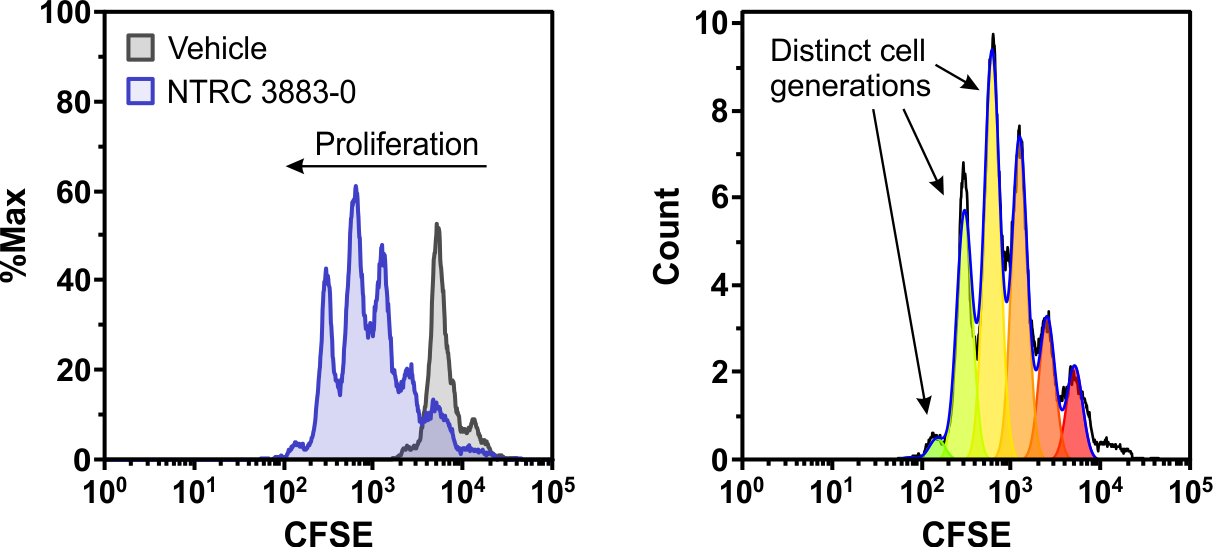
January 28, 2021: A new article on the role of tryptophan-metabolizing enzymes in cancer immunology is now online in Frontiers in Immunology (Grobben et al., 2021). The figure shows a co-culture assay of IDO1-overexpressing HEK-293 cells with lymphocytes from a healthy donor. NTRC 3883-0 restores proliferation of the fluorescently labeled CD8-positive T cells (left), as indicated by the multiple cell generations present in the T cell population (right).
New article in Frontiers in Immunology sheds light on role of tryptophan-metabolizing enzymes in cancer immunology
Oss, January 28, 2021, – The amino acid L-tryptophan is a key regulator of the immune modulatory activity of T cells and natural killer cells. L-tryptophan-catabolizing enzymes are therefore considered as important targets for cancer immunotherapy. However, the phase III clinical failure of the selective IDO1 inhibitor epacadostat (Long et al., 2019), as well as negative clinical results of other IDO1 inhibitors (Jung et al., 2019; Reardon et al., 2020), raised doubt about the validity of IDO1 as a drug target for cancer immunotherapy. In a new article in the open access journal Frontiers in Immunology, researchers from NTRC (Grobben et al., 2021) shed light on the role of IDO1 in models of cancer immunotherapy on the basis of the small molecule IDO1 inhibitor NTRC 3883-0. This compound is derived from a hit identified in a screen performed by the European Lead Factory, and has a chemical structure distinct from previously described IDO1 inhibitors (de Man et al. 2018). NTRC 3883-0 was profiled alongside epacadostat in a variety of biochemical and cell-based assays (see illustration above) and two syngeneic mouse models: the often-used colon carcinoma CT26 model (Koblish et al., 2010), and a melanoma model using IDO1-overexpressing B16F10 cells (Holmgaard et al. 2015).
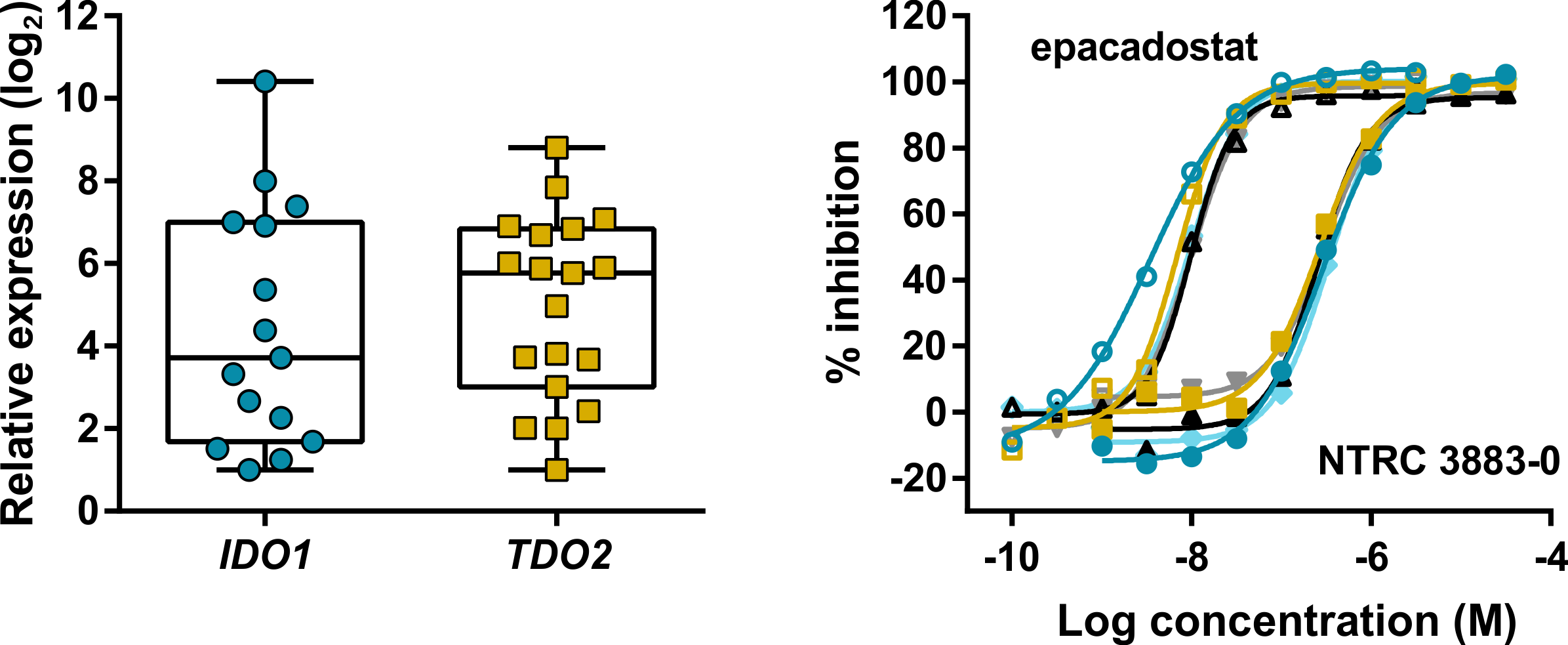
Expression and modulation of IDO1 and TDO in adherent cell samples isolated from the ascites of ovarian cancer patients.
Left: Tukey boxplot of basal IDO1 and TDO2 gene expression as determined by qPCR in adherent cell samples cultured for two to three passages.
Right: Inhibition of the Trp-catabolizing activity in IFNγ-stimulated samples by NTRC 3883-0 (closed symbols) and epacadostat (open symbols).
Grobben and colleagues also demonstrate for the first time the use of primary ovarian cancer cell cultures isolated from ascites of ovarian cancer patients to determine IDO1 activity. Ascites is the abnormal build-up of fluid in the peritoneal cavity. Many patients with ovarian cancer present with ascites at diagnosis, which is therefore a potential source of clinical biomarkers. The use of cells from ascites has a major advantage over surgical biopsies or organoid cultures, because the collection of ascites is minimally invasive and assays to determine the activity of compounds can be performed ex vivo prior to the start of therapy (den Ouden et al., 2020).
Notably, in all nineteen primary ovarian cancer cell cultures investigated in the study, expression of TDO was detected. TDO catalyses the same enzymatic conversion of L-tryptophan as IDO1 but is evolutionary unrelated and has a different tissue distribution. Following the clinical failure of epacadostat, it has been argued that a dual IDO1/TDO inhibitor may be preferred over a selective IDO1 inhibitor to regulate cancer immune response (Prendergast et al., 2017). These and other relevant topics related to tryptophan-metabolizing enzymes in cancer and their application as small molecule drug targets for cancer immunotherapy are discussed in the article.
NTRC is a precision medicine company dedicated to discovering new anti cancer drug candidates. We help you to find a mechanistic hypothesis before entering the clinic. We can study a wide range of cancer cells, primary patient material and immune cells in vitro, in isolation and in coculture, after exposure to monotherapy and combination therapy. In addition, we perform in-depth mechanistic analyses in cells and by biophysical methods, such as Biacore and LC-MS/MS. Keywords are: Quality. Flexibility. Short Turnaround Time.





