Comparative biochemical kinase profiling of VEGFR2 inhibitors
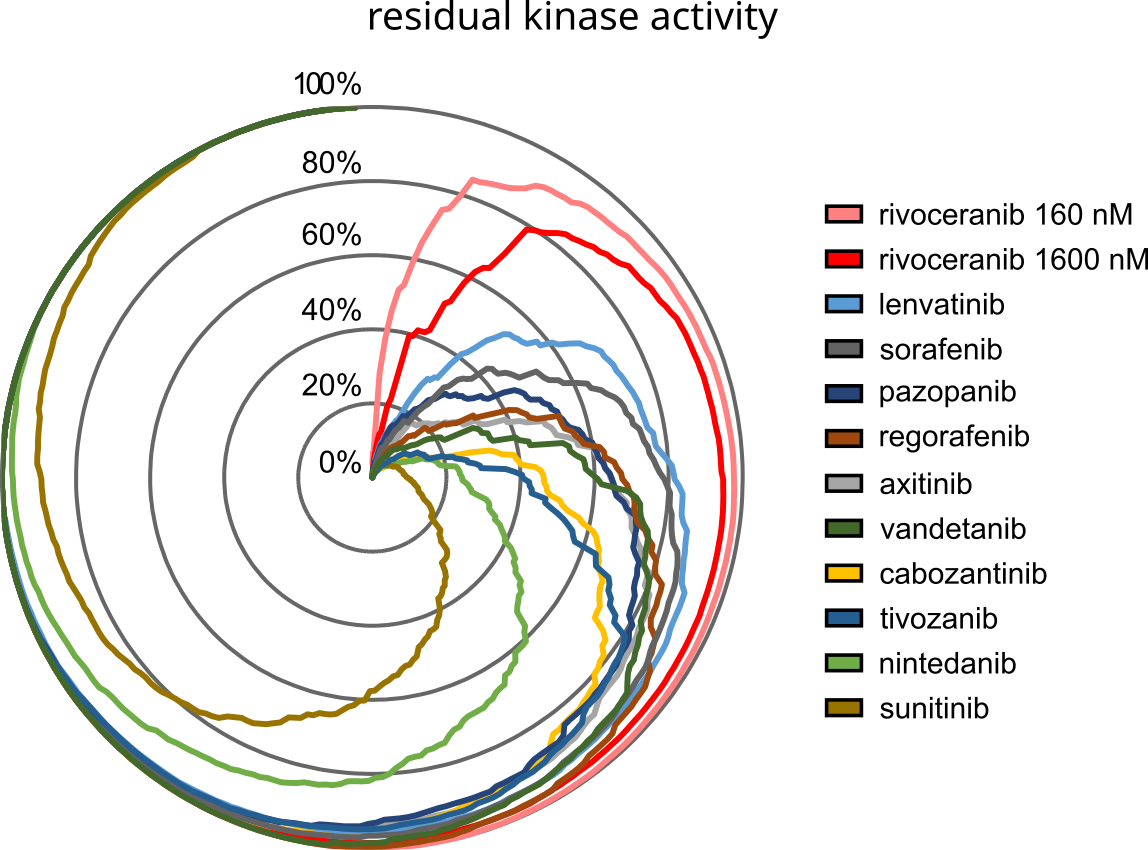
Figure. Radar plot showing residual activity of 270 kinases in the presence of rivoceranib (160 or 1600 nM) or 1000 nM of 10 FDA-approved reference tyrosine kinase inhibitors.
Vascular endothelial growth factor receptor 2 (VEGFR2) is a key regulator of tumor angiogenesis, the formation of new blood vessels from existing vasculature. VEGFR2 has been an attractive target for anti-cancer therapy. However, clinical application of available VEGR2 inhibitors has been challenged by limited efficacy and a wide range of side effects, potentially due to inadequate selectivity for VEGFR2.
In a study co-authored by scientists from Elevar Therapeutics and Oncolines in Cancer Chemotherapy and Pharmacology [1], the biochemical kinase activity profiles of eleven small molecule tyrosine kinase inhibitors are compared on VEGFR2 and 270 kinases representative of the human kinome. Rivoceranib [2], a kinase inhibitor under review at the U.S. Food & Drug Administration (FDA), was identified as a highly selective VEGFR2 inhibitor. The comparative biochemical analysis highlights the potential for rivoceranib to address clinical limitations associated with off-target effects of currently available VEGFR2 inhibitors.
The study highlights the power of combining Oncolines’ ResidenceTimer platform and Carna Bioscience’s kinase activity profiling.
References
Oncolines B.V. is a precision medicine services company in oncology and cancer immunotherapy. Oncolines is part of the Symeres group of companies, a group of high-quality CROs and CDMOs based in Europe and the United States.





