First presentation of novel mitotic checkpoint inhibitor BAL0891 data at ESMO TAT congress 2022
At the virtual Targeted Anticancer Therapy (TAT) congress, which was organized by the European Society of Medical Oncology (ESMO) on 7-8 March, Basilea Pharmaceutica International Ltd. and Netherlands Translational Research Center B.V. (NTRC) have, for the first time, presented preclinical in vitro and in vivo data of BAL0891, a dual TTK/PLK1 inhibitor with potent single agent activity.
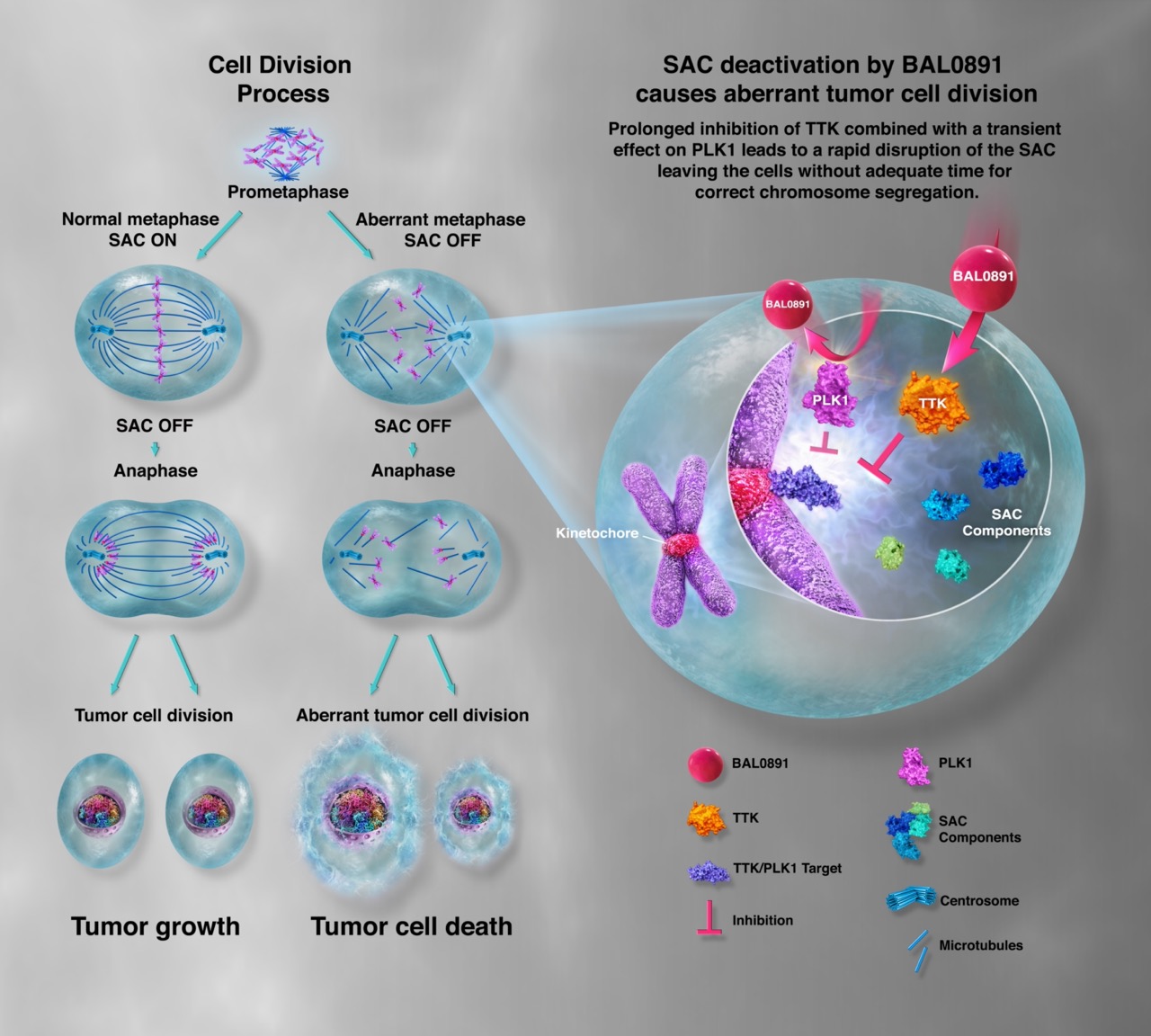
Figure. Schematic representation of mechanism of action of BAL0891. Long target residency on TTK combined with a transient effect on PLK1 leads to a rapid disruption of the SAC, leaving tumor cells without adequate time for correct chromosome segregation. – Copyright of Basilea Pharmaceutica (Basel, Switzerland).
At the congress, Dr. Heidi Lane from Basilea showed that single agent therapy with BAL0891 in mouse models of human cancer results in tumor regression and confirmed cures [1]. Potent single agent activity is ascribed to its dual targeting of Threonine Tyrosine kinase (TTK) and a second mitotic kinase, Polo-like kinase 1 (PLK1), which cooperates together in the Spindle Assembly Checkpoint (SAC) regulation [2]. The Figure shows the proposed mechanism of action of BAL0891 in more details.
The spindle assembly checkpoint (SAC) is a surveillance mechanism that controls the correct segregation of sister chromatids during cell division. This process is regulated through the activity of a number of protein kinases, including TTK, also known as Mps1. Treatment of cancer cells with small molecule inhibitors of the SAC results in chromosome mis-segregation, driving these cells in aberrant mitosis and cell death.
BAL0891 was discovered in an optimization program focusing on long target residence time on TTK at NTRC [3]. Biomarkers which potentially could be useful for patient stratification for treatment with TTK inhibitors were identified by Oncolines™ cancer cell panel profiling [4] and mechanistic cell biology [5]. The compound was further developed by Basilea after licensing the project from NTRC in 2018. In December 2021 the U.S. Food & Drug Administration (FDA) approved Basilea’s Investigational New Drug (IND) application of BAL0891 and preparations are currently ongoing to enable the start of a Phase 1 clinical trial in patients with advanced solid tumors mid-2022. (see press release)
Overall, the contribution from both cancer research groups at NTRC and Basilea have been key to support the IND application for BAL0891. ResidenceTimer™ and Oncolines™ Profiling & Bioinformatics, two technology platforms that have been instrumental to the discovery and development of BAL0891, are available as fee-for-services at Oncolines B.V., a spin-off company of NTRC.
References[1] Lane et al. (2022) BAL0891: a novel, small molecule, dual TTK/PLK1 mitotic checkpoint inhibitor (MCI) with potent single agent activity. ESMO TAT presentation number 42P[2] von Schubert et al. (2015) Mps1 and Plk1 cooperatively regulate the spindle assembly checkpoint in human cells. Cell Reports 12, 66-78.[3] Uitdehaag et al. (2017) Target residence time-guided optimization on TTK kinase results in inhibitors with potent anti-proliferative activity. Journal of Molecular Biology 429, 2211-30.[4] Zaman et al. (2017) TTK inhibitors as a targeted therapy for CTNNB1 (β-catenin) mutant cancers. Molecular Cancer Therapeutics 16, 2609-17.[5] Libouban et al. (2017) Stable aneuploid tumor cells are more sensitive to TTK inhibition than chromosomally unstable cell lines. Oncotarget 8, 38309-25.
About Oncolines
Oncolines B.V. is a precision medicine services company in oncology and cancer immunotherapy. We help to bring improved and novel therapies to the right patient population faster. We offer a set of complimentary services to enable our clients to characterize their compounds, and to determine activities, selectivities and mechanism of action. We present results in a unique and interactive reporting format that facilitates easier and faster interpretation of results.