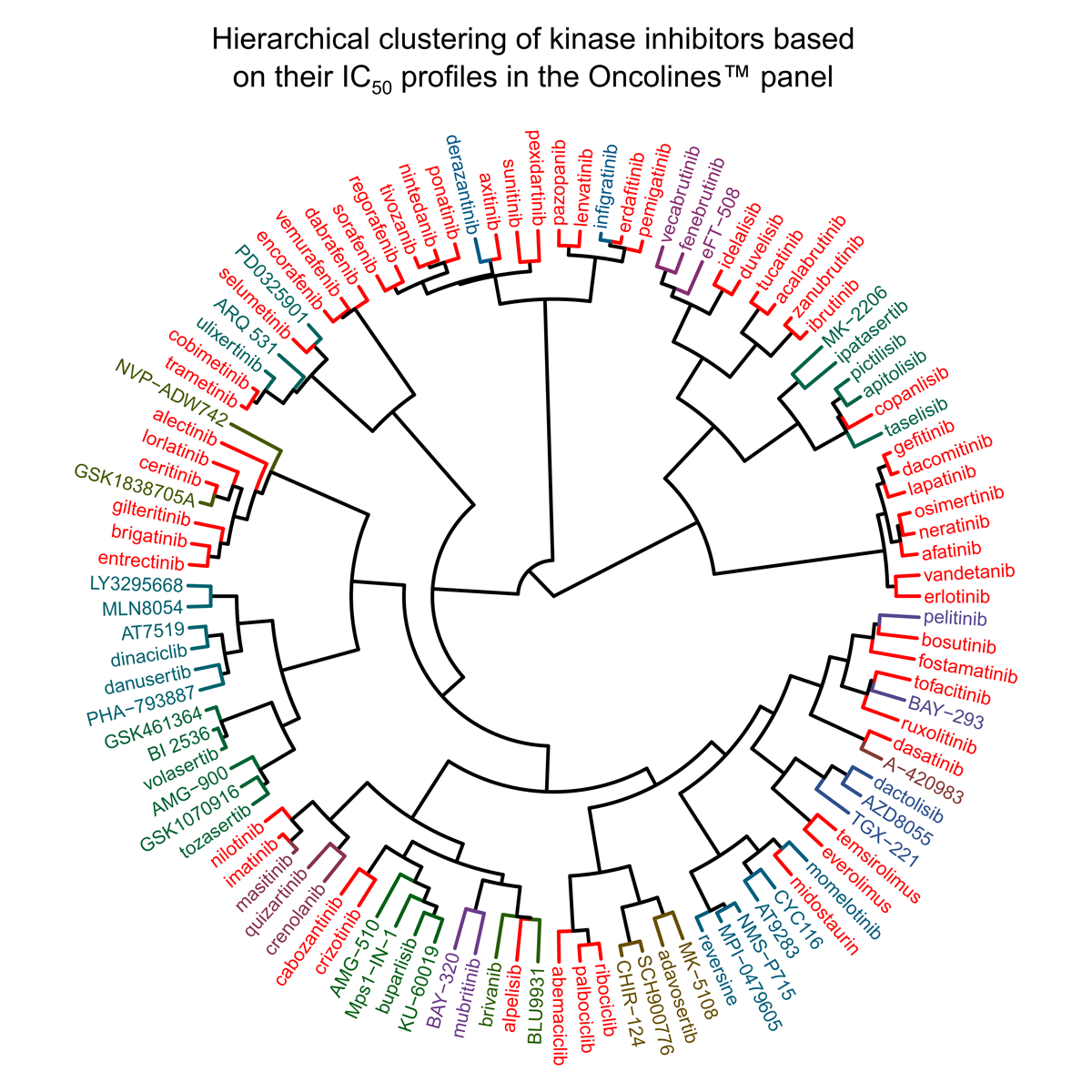
September 14, 2020: Hierarchical clustering of kinase inhibitors based on their IC50 profiles in the Oncolines™ panel. Names of approved kinase inhibitors are given in red.
Clustering analysis of recently approved kinase inhibitors (2018-2020)
Oss, September, 14, 2020 – The 18th Discovery on Target (DOT) conference on innovative drug discovery will be in digital format this year. Dr. Guido Zaman, Managing Director and Head of Biology of NTRC, will present at the Kinase Inhibitor Discovery Symposium, held on Thursday and Friday, September 17th and 18th.
Recently, the first tumor-agnostic kinase inhibitors, targeting NTRK gene fusions in solid tumors, and several new mechanisms have been addressed, including selective targeting of FGFR and RET. The presentation concerns clustering analysis of recently approved kinase inhibitors (2018 – 2020) on the basis of profiling in a broad cancer cell line panel (Oncolines™). The work proceeds on the work of two earlier published studies [1,2] on clinically approved kinase inhibitors. So far, more than hundred kinase inhibitors have been profiled in the Oncolines™ cancer cell line panel. Approximately half of them are clinically approved drugs (indicated in red in the clustering wheel). The selectivity of these compounds on a panel of 280 biochemical kinase enzyme assays have also been determined (at Carna Biosciences, Inc.). In the presentation at the DOT virtual conference, Guido Zaman will relate the biochemical and the cellular selectivity of recently approved kinase inhibitors, including inhibitors of BRAF, FLT3 and FGFR. All case studies are new and have not been presented or published before.
Discovery on Target Kinase Inhibitor Symposium. Title of presentation: “Biochemical and cellular selectivity of recently approved kinase inhibitors” Time: 2.05 p.m. (EDT) / 8.05 p.m. (CET).
References:
NTRC is a precision medicine company dedicated to discovering new anti cancer drug candidates. We help you to find a mechanistic hypothesis before entering the clinic. We can study a wide range of cancer cells, primary patient material and immune cells in vitro, in isolation and in coculture, after exposure to monotherapy and combination therapy. In addition, we perform in-depth mechanistic analyses in cells and by biophysical methods, such as Biacore and LC-MS/MS. Keywords are: Quality. Flexibility. Short Turnaround Time.





