NTRC and Radboud explore new methods to predict chemotherapy response in ovarian cancer
Oss, March, 27th, 2019 – NTRC and Radboudumc Nijmegen (The Netherlands) are developing a platform to predict the response of high-grade serous ovarian cancer patients to chemotherapy by using tumor and immune cells isolated at diagnosis. While patients receive normal standard-of-care treatment, data are collected to improve future diagnostics and care. In particular, genomic and immune cell biomarkers are validated that may predict patients’ response to classic chemotherapy, as well as predictive drug response biomarkers for novel targeted drugs and immune therapies. Progress on the development of a collection of patient cell-derived functional assays will be presented at the Annual meeting of the American Association of Cancer Research (AACR) in Atlanta (United States) April 1st (Abstract #2221).
Epithelial ovarian cancer is the most lethal gynaecologic malignancy worldwide. First-line treatment is surgery in combination with chemotherapy consisting of a platinum-based drug and paclitaxel. Although most women show a good response to this treatment, tumors do not respond in 15-20% of patients, whereas in 80% of cases of advanced ovarian cancer, the disease recurs within three years. Ovarian cancers with mutations in DNA repair genes, such as BRCA1 or 2, are more sensitive to platinum therapy and patients have longer overall survival [1]. Inhibitors of PARP enzymes prevent DNA repair pathways and synergize with platinum therapy [2]. Four PARP inhibitors have been approved for clinical use in second line or maintenance therapy of platinum sensitive epithelial ovarian cancer. At the moment, BRCA gene mutations for usage of PARP inhibitors is the only approved companion diagnostic test in ovarian cancer therapy.
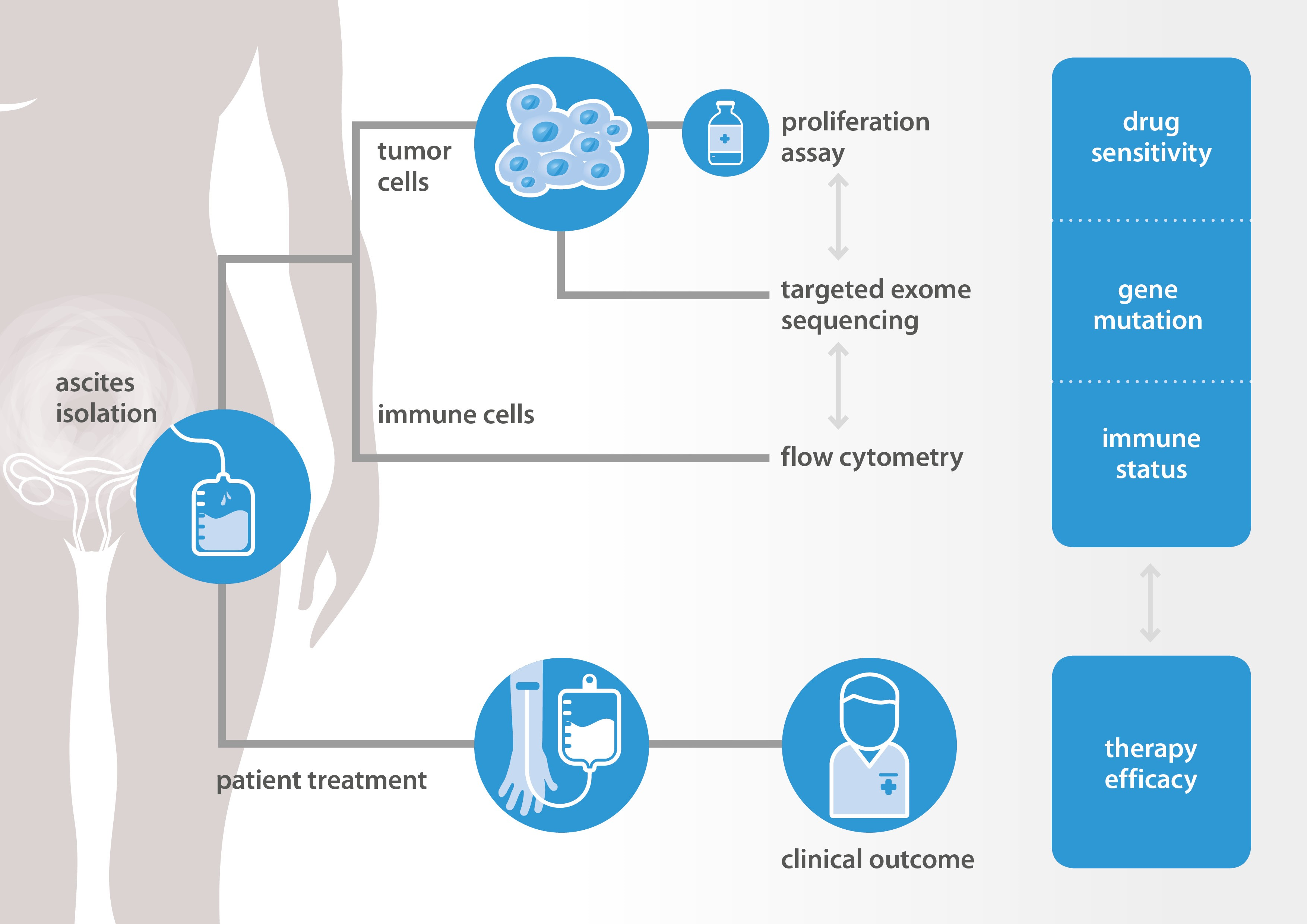
New methods are needed to select the right drugs for the right patients. NTRC and Radboudumc explore new methods to predict response to therapy in ovarian cancer. Data are collected and combined from in vitro tumor cell proliferation assays, DNA sequencing of cancer and DNA repair genes, and immunological assays. All in vitro assays are performed at NTRC and results are compared to tumor histopathology and clinical response data collected at Radboudumc. A schematic workflow of the project is presented in the Figure below.
At the AACR 2019 conference, the feasibility to perform proliferation assays with patient-derived tumor cells will be presented. A differential response of a targeted kinase inhibitor could be related to a cancer driver gene mutation that was previously identified as a predictive drug response biomarker by profiling on NTRC’s Oncolines™ cancer cell line panel [3]. Immune cell characterization and expression analysis of immune markers on tumor cells were also performed. These ‘immune markers’ may also provide new predictive or prognostic biomarkers in epithelial ovarian cancer [4].
In an ongoing study of NTRC, Radboud and several other hospitals in The Netherlands, hundred patients with high-grade serous ovarian cancer will be included and in vitro drug response of tumor cells from ascites will be determined. Results will be related to gene mutations, immune status and clinical response data. Predictive drug response biomarkers identified by cancer cell panel profiling in NTRC’s Oncolines™ panel will be validated using primary patient-derived materials.
Liturature references
[1] Pennington et al. (2014) Clin Cancer Res 20, 764-775.[2] Konstantinopoulos et al. (2015) Cancer Discov 5, 1137-1154[3] Uitdehaag et al. (2019) Mol Cancer Ther 18, 470-481[4] Curiel et al. (2004) Nature Med 10, 942-949
NTRC is a precision medicine company dedicated to the development of new anti-cancer drugs. NTRC facilitates the development of novel therapies by providing custom-based assays with primary patient material and high-throughput cancer cell line profiling services (Oncolines™, GeneNominator™, SynergyFinder™ and SynergyScreen™), as well as target residence time measurements for protein kinases (ResidenceTimer™) on a fee-for-service basis. Arginase Gold™ and NFK GreenScreen™ are assay read-outs for the cancer immunotherapy drug targets Arginase-1, and IDO1 and TDO respectively. Assay kits are supplied to clients globally.